A Versatile Approach to the Synthesis of Polyphosphazene Derivatives via the ThiolĘCEne Reaction |
| Timeú║2012-12-19 15:03 |
Yue-Cheng Qian, Xiao-Jun Huang*, Chen Chen, Ning Ren, Xu Huang, Zhi-Kang Xu; J. Polym. Sci., Part A: Polym. Chem. 2012, 50: 5170-5176
http://onlinelibrary.wiley.com/doi/10.1002/pola.26361/abstract
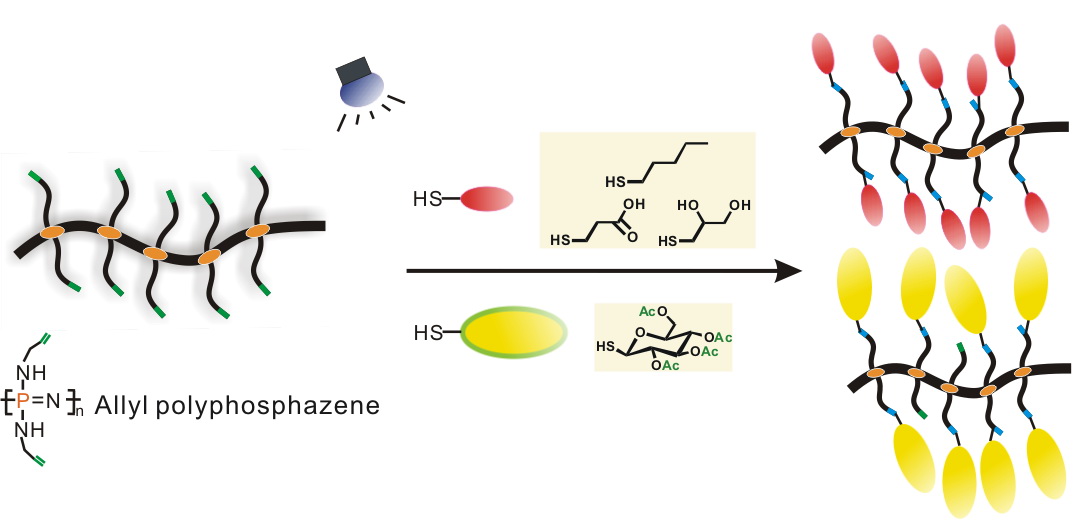
The thiol–ene radical addition reaction has been successfully used to synthesize polyphosphazene derivatives. Poly[bis(allylamino)phosphazene] with pendant allyl groups was reacted with different thiol reagents under UV irradiation. These thiol reagents include 1-pentanethiol, 3-mercaptopropionic acid, 3-mercapto-1,2-propane-diol, and 2,3,4,6-tetra-O-acetyl-1-thio-β-d-glucopyranose. 1H NMR analyses confirm that the allyl polyphosphazene can be quantitatively modified by the mercaptans. In total, 100% conversion of the allyl groups was reached in <60 min toward the first three mercaptans, whereas about 80% conversion of the allyl groups was reached after 120-min reaction toward the thioglucose. This method is a facile route for the synthesis of functional polyphosphazenes without the needs for protection/deprotection procedures.
|
Readú║2087
|
