|
当前位置:他山之石 -> 聚合物能把游泳池变成果冻
Polymer can turn swimming pool to jelly
聚合物能把游泳池变成果冻
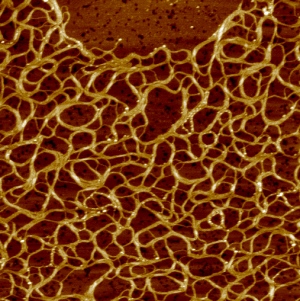
“受热时,这种新的聚合物就形成网络。网络的间隙大约十分之一微米。用一千克这种聚异腈高分子,挥洒到一个奥林匹克游泳池中,轻轻加热,数秒之后,果冻就形成了。”
荷兰Radboud大学的材料化学家Alan Rowan正在讲述他们实验室制备的令人惊奇的高分子材料。他并没有真的做这个把游泳池变成果冻的实验,不过他声称他倒是愿意试一下。他兴冲冲地说,如果说到形成凝胶,他的聚合物是世界上最好的——比其它任何东西都好十倍。
When warmed, a new synthetic polymer forms a network, seen here under an atomic force microscope. The gaps are about one-tenth of a micrometer.
Alan Rowan, a materials chemist at Radboud University Nijmegen in the Netherlands, is describing the properties of a remarkable polymer developed in his lab and unveiled today in Nature1. He has not actually run the swimming-pool experiment, but he sounds as if he would love to give it a try. When it comes to forming gels, he says excitedly, his polymer is “probably the best in the world — an order of magnitude better than anything else”.
然而,这远比提供一个破纪录的餐后甜点要多得多。芝加哥大学的生物物理学家Margaret Gardel如是说。这是第一种能够与天然高分子的刚性媲美的合成高分子。“几乎所有的天然高分子,就像DNA和胶原蛋白那样,都有一些内禀的刚性”,她解释道。相反,合成高分子则过于松软。
But it offers much more than record-breaking dessert portions: it is the first synthetic polymer that can match the rigidity found in many biological polymers, says Margaret Gardel, a biophysicist at the University of Chicago, Illinois, who wrote a News and Views article2 to accompany the publication. “Nearly all biopolymers, like DNA or collagen, have some inherent rigidity,”she explains; synthetic polymers, by contrast, tend to be extremely floppy.
Rowan 制备的高分子链具有螺旋状骨架,带着成千上万的伸向骨架旁边的短肽,每个短肽都带着一条由碳和氧原子构成的长尾巴。相邻肽上的氧和氢原子相互键连赋予主链刚性,而由碳和氧构成的尾端便于抓住水分子,使聚合物极易溶解。
Rowan’s polymer strands have a helical backbone with thousands of short peptides jutting out from the sides, each carrying long tails made of repeating carbon and oxygen chains. Oxygen and hydrogen atoms in neighbouring peptides bond to each other to give the backbone rigidity, and the carbon and oxygen tails readily grab water molecules, making the polymer extremely soluble.
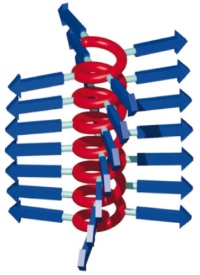
聚合物具有螺旋结构的主链(红色)和连接相邻聚合物链的侧链(蓝色)
The polymer has a helical backbone (red) and tails (blue) that link to neighbouring strands.
强有力的结构Strong structure
一旦聚合物溶解,加热就能引起尾端把水分子排挤出去并与相邻高分子链形成连接。在某个温度之上,溶液数秒之内就转变成凝胶,因为聚合物链自组装成粗细10纳米左右的集束。就像或的细胞中的生物大分子或者绳子中的纤维,这些集束硬化了整个结构。纳米尺度上的微观机制与宏观尺度一样。Rowan说。
Once the polymer is dissolved, warming it causes the tails to squeeze water molecules away and form links with neighbouring polymer strands. Above a certain temperature, the solution transforms into a gel in seconds as the strands self-assemble into bundles roughly 10 nanometres wide. As with the biopolymers in a living cell, or the fibres in a rope, the bundling stiffens the whole structure. “The nanoscale mechanism is the same as at the macroscale,” says Rowan.
研究者们早已知道集束在生物高分子强化中的重要性。但Rowan的研究队伍测定了单个高分子链和单个集束的强韧性,并研究了二者的关系。Rowan预言:“如今,我们既然知道了原理,那就可以用浓度更低的聚合物制备凝胶了”。
Researchers already knew that bundling was important in strengthening biopolymers. But Rowan’s team has measured the stiffness of individual strands and of the bundles, and has shown the relationship between the two. “Now that we understand the principles, we can start making gels at even lower concentrations,” predicts Rowan.
聚合物受热形成凝胶的能力是非同寻常的:对大多数凝胶而言,反过来也是如此。例如,一种溶胶必须冷却才能形成冻胶。Rowan设想把他聚合物的凉溶液注入到伤口处,然后在体温的加热下形成凝胶壁垒以保护组织;不再需要时,就用一个冰袋冷敷一下,凝胶就液化了。研究者们正在验证这个理念。
The polymer’s ability to form a gel on heating is relatively unusual: the opposite is true for most gels. A gelatin solution, for example, must be cooled to form jelly. Rowan imagines that a cold solution of his polymer poured on to a wound to protect the tissue would quickly form a gel barrier when it warmed to body temperature; applying an ice pack could liquefy it when it was no longer needed. The researchers are now running tests on this concept, “using a leg of pork that we put in the oven at 40 °C”, says Rowan.
Corrections
A previous version of this article stated that nitrogen and hydrogen atoms in neighbouring peptides bond to each other, but it is oxygen and hydrogen atoms that do the bonding.
1. References
2. Kouwer, P. H. J. et al. Nature, http://dx.doi.org/10.1038/nature11839 (2013).
3. Gardel, M. L. Nature http://dx.doi.org/10.1038/nature11855 (2013).
返回>>他山之石 |