News
| Phenomenon and Mechanism of Capsule Shrinking in Alkaline Solution Containing Calcium Ions |
| Time:2012-12-17 15:09 Source:未知 Author:polymer Click: |
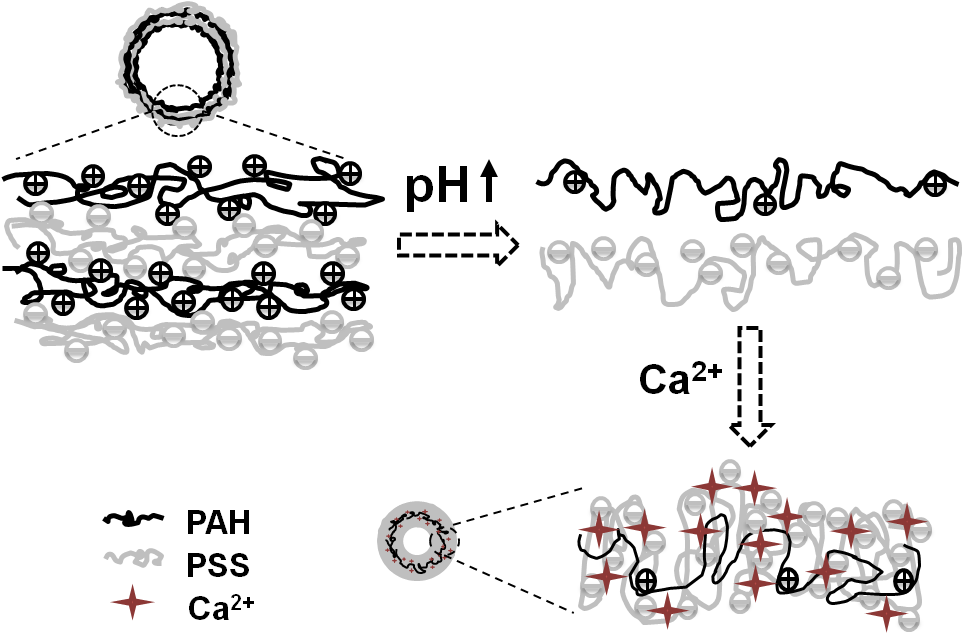
Shrinking phenomenon of poly(allylamine hydrochloride) (PAH)/poly(styrene sulfonate, sodium salt)(PSS) multilayer microcapsules was observed when they were incubated in alkaline solutions containing Ca2+. The shrinking was universal to those polyelectrolyte multilayer capsules regardless of the wall thickness and wall compositions suppose the conditions were proper. The shrinking extent increased along with the increase of solution pH and Ca2+ concentration, and reached to a maximum value of 70% (from 7.4 to 2.3 μm). The shrunk capsules with a hollow structure and thick wall could well maintain their spherical shape in a dry state. During the capsule shrinking partial loss of the polyelectrolytes especially PSS took place, and the loss amount increased along with the increase of solution pH although the alteration patterns were different at lower Ca2+ concentration. The complexation of PSS with Ca2+, which is believed one of the major reasons governing the capsule shrinking, was demonstrated by X-ray photoelectron spectroscopy and turbidity experiment. The mechanism is proposed, which relies on the synergistic effects of deprotonation of PAH and screening of PSS by Ca2+ leading to the thermodynamically favored-capsule shrinking.
|